Downloading Content for Analysis
This page is recommended for advanced users. It explains how to download study record data in Extensible Markup Language (XML), a machine-readable format, and in other data formats.
Use of ClinicalTrials.gov data is subject to these Terms and Conditions.
Contents
- Download Study Information from the Search Results List
- ClinicalTrials.gov Application Programming Interface (API)
- Download All Study Record Content for Analysis
- Access or Download the Clinical Trials Transformation Initiative (CTTI)'s Database for Aggregate Analysis of ClinicalTrials.gov (AACT)
Download Study Information from the Search Results List
The Download option on the Search Results page is an advanced feature that allows you to download information about some or all of the studies shown in the search results:
Click on the Download link (shown above), which is located in the upper-right corner of the List tab on the Search Results page. A pop-up box containing download options will appear:
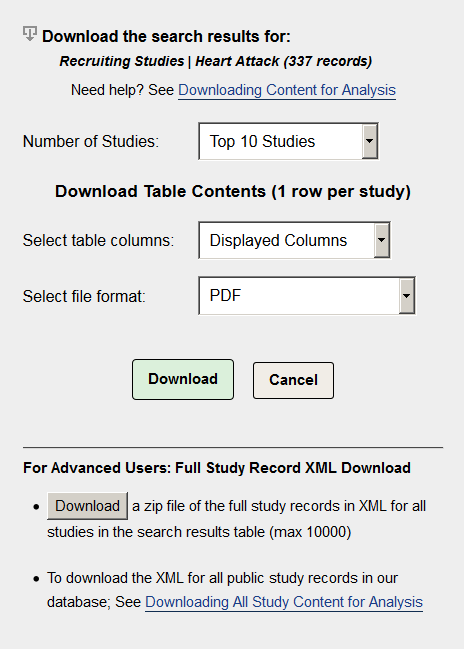
Number of Studies: Choose the number of studies you want to download using the dropdown list. Your options are:
- The top 10, 100, 1,000 or 10,000 studies retrieved by your search. These options will be available only for searches that find as many or more than the specified number of studies. For example, if your search finds fewer than 1,000 studies, "Top 10 Studies" and "Top 100 Studies" can be selected, but "Top 1000 Studies" will not appear in the dropdown list.
- All studies retrieved by your search (up to a maximum of 10,000 study records). For example, if your search finds 54 studies, "54 Found Studies" will appear in the dropdown list.
Download Table Contents (1 row per study):
Select Table Columns
Use the dropdown menu to choose which table columns are downloaded for each study and in what format:
- Displayed Columns. Choose this option to download only table columns shown onscreen. The default study columns shown onscreen are Row, Status, Study Title, Condition and Interventions. To change which columns are shown in your search results, close the window you are in, click on the Show/Hide Columns link (located on the right side of the search results List tab), and then add or remove columns by marking or unmarking the column names.
- All Available Columns. Choose this option to download all available table columns. Includes over 20 columns such as Status, Conditions, Interventions, Study Type, Phase, and Sponsor/Collaborators. For more information about columns, see Customize Your Search Results Display.
Select file format.
Use the dropdown list to select one of the following formats for your saved file:
- PDF. Save as Portable Document Format (PDF), a standard format accessible using the freely available Adobe Acrobat Reader DC.
- Plain text. Save as unformatted text that can be read in a simple editor, such as Notepad.
- Tab-separated values. Save each study as a separate line in the file, with tabs as delimiters, or spacers, between each field. This format is useful for importing study information into spreadsheets and databases.
- Comma-separated values. Save each study as a separate line in the file, with commas as delimiters, or spacers, between each field. This format is useful for importing study information into spreadsheets and databases.
- XML. Save as XML, a machine-readable format that will be most useful to advanced users.
Click on the Download button directly beneath the "Select file format" drop-down menu to save this file to your computer.
For Advanced Users - Download XML Study Record Content.
Click on the Download button under "For Advanced Users:" to save the complete XML for all study records (that is, all registration information as well as any available results information) retrieved by your search (up to a maximum of 1,000 study records) as a zip file or compressed package to your computer. (Sample zip file readers with free trial periods: WinZip and PKZip)
Note: It may take several minutes to download a large number of studies.
To download XML for more than 1,000 study records in a single zip file, see the next section on Use URL Parameters to Display and Save Data.
ClinicalTrials.gov Application Programming Interface (API)
The ClinicalTrials.gov Application Programming Interface (API) provides a toolbox for programmers and other technical users to access publicly accessible information for all study records posted on ClinicalTrials.gov. For example, the API can be used to encode a search query as a URL. Clicking on the URL activates the query to search and retrieve study records from ClinicalTrials.gov.
https://ClinicalTrials.gov/api/query/full_studies?expr=heart+attack
The structure of study records in XML is defined by this XML schema. [The ClinicalTrials.gov registration and results data elements with their corresponding API fields are listed in these crosswalks.]
Note: Beginning on 1/1/2022, the previous API that uses this XML schema will no longer be supported.
Downloading All Study Record Content for Analysis
A single zip file containing all study records available on ClinicalTrials.gov (that is, all registration information as well as any available results information for all study records) in XML format is available here:
- To download the zip file containing all public data, enter the following URL into your browser:
https://classic.clinicaltrials.gov/AllPublicXML.zip
Note: This is a very large file. It will likely take several minutes to download the entire zip file. Additionally, many receiving systems may subject the zip file to automatic security/virus scanning. This scanning may take several additional minutes to complete before the zip file is ready for use. Please be patient.
The zip file contains multiple directories subdividing the large number of files into more manageable quantities. A top level "Contents.txt" file contains information about the number of study records and the date the studies were published.
Access or Download the Clinical Trials Transformation Initiative (CTTI)'s Database for Aggregate Analysis of ClinicalTrials.gov (AACT)
CTTI, a public-private partnership, has restructured and reformatted ClinicalTrials.gov data into a relational database under its State of Clinical Trials project.
The CTTI Improving Public Access to Aggregate Content of ClinicalTrials.gov page provides instructions on accessing the database directly or downloading a static snapshot.
Supporting information, such as a data dictionary, database schema, and a guide for researchers and analysts, are also available on the AACT database page.


